Covid 19 diagnostic device applications received by health canada device name manufacturer device type footnote 1 laboratory or point of care test footnote 1 status.
Point of care devices for covid.
They cannot test saliva or blood.
Press release public statement.
The assure covid 19 igg igm rapid.
All three of these tests require nasal or throat specimens on swabs.
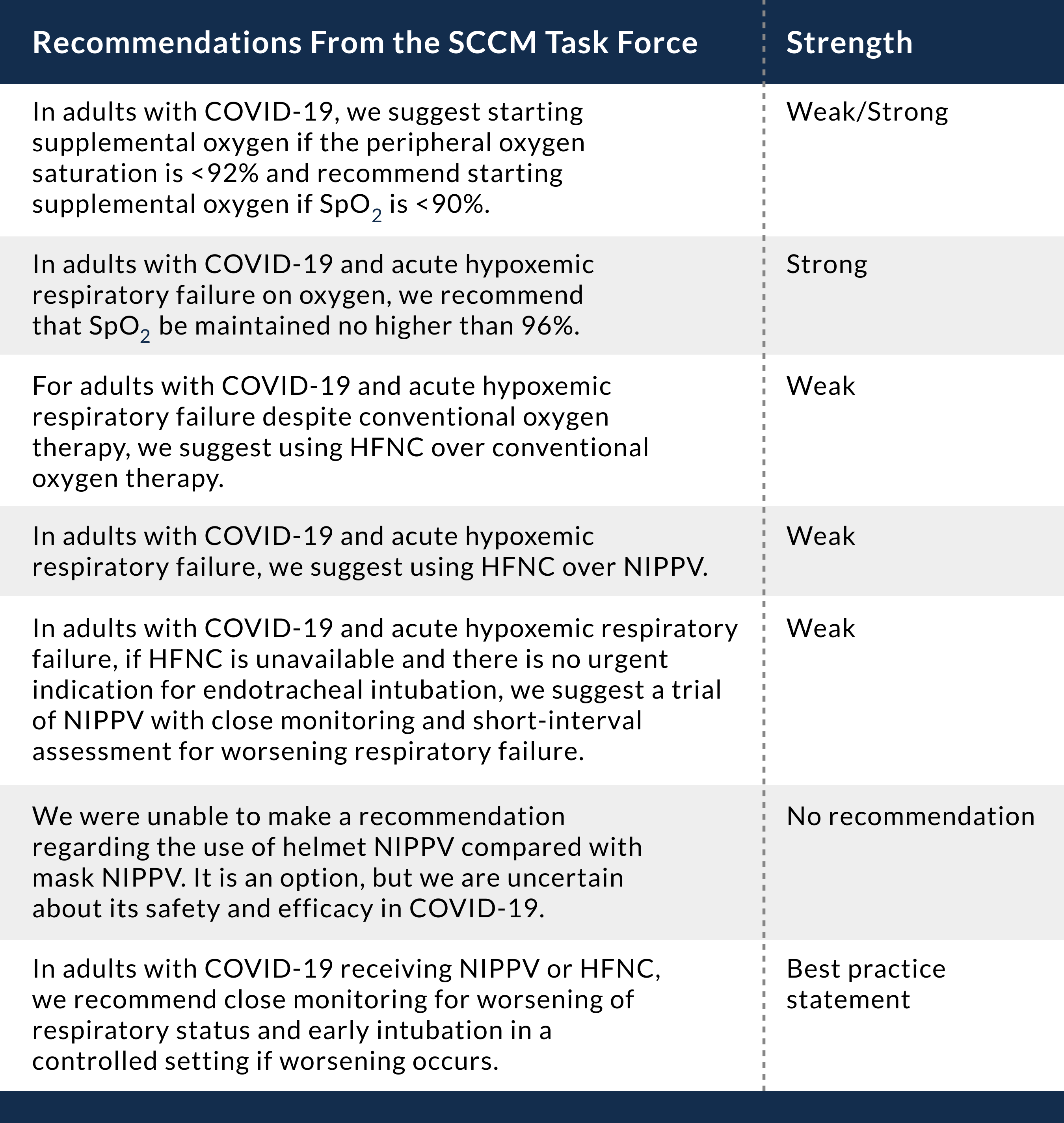
As of april 29 fda has granted emergency use authorization for three commercial products with covid 19 tests that can be run in patient care settings i e.
Thousands of nursing homes across the country will be given point of care covid 19 tests by the administration starting next week officials announced tuesday.
Quidel sofia 2 sars antigen fia.
Press release point of care testing poct devices market analysis 2020 covid 19 impact and global analysis by key companies comprehensive analysis by global industry share size growth.
Point of care reverse pcr tests as of april 29 fda has granted emergency use authorization for three commercial products with covid 19 tests that can be run in patient care settings i e.
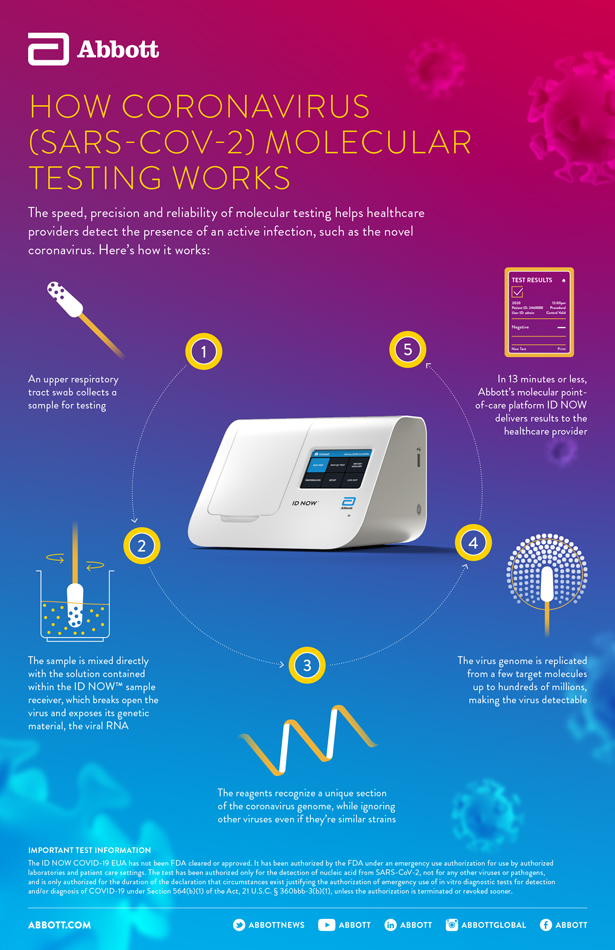
The id now covid 19 rapid test delivers high quality molecular positive results in as little as 5 minutes targeting the coronavirus covid 19 rdrp gene.
Pathogendx united states.
Point of care reverse pcr tests.
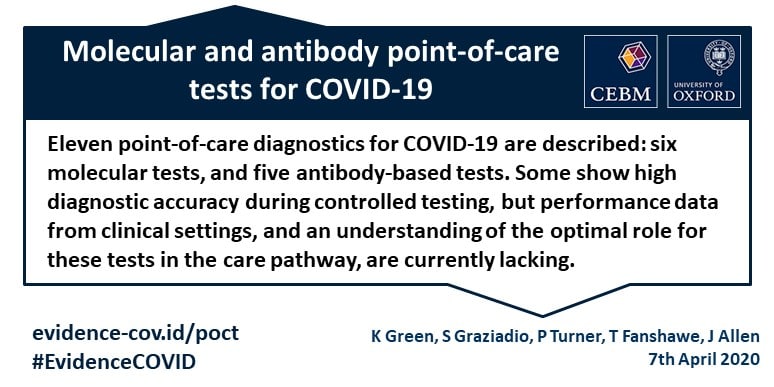
Point of care tests.
South korea antigen technology.
Nursing facilities will receive one of two testing devices.
All three of these tests require nasal or throat specimens on swabs.
They cannot test saliva or blood.
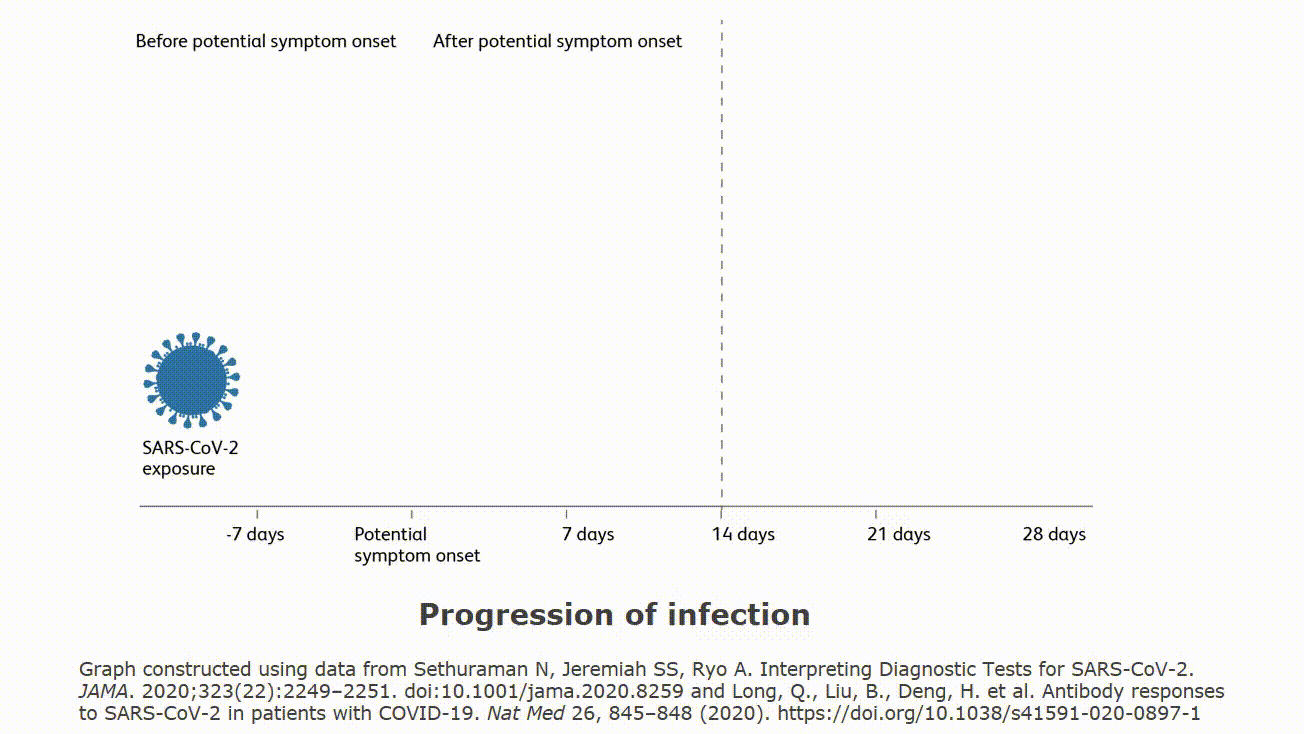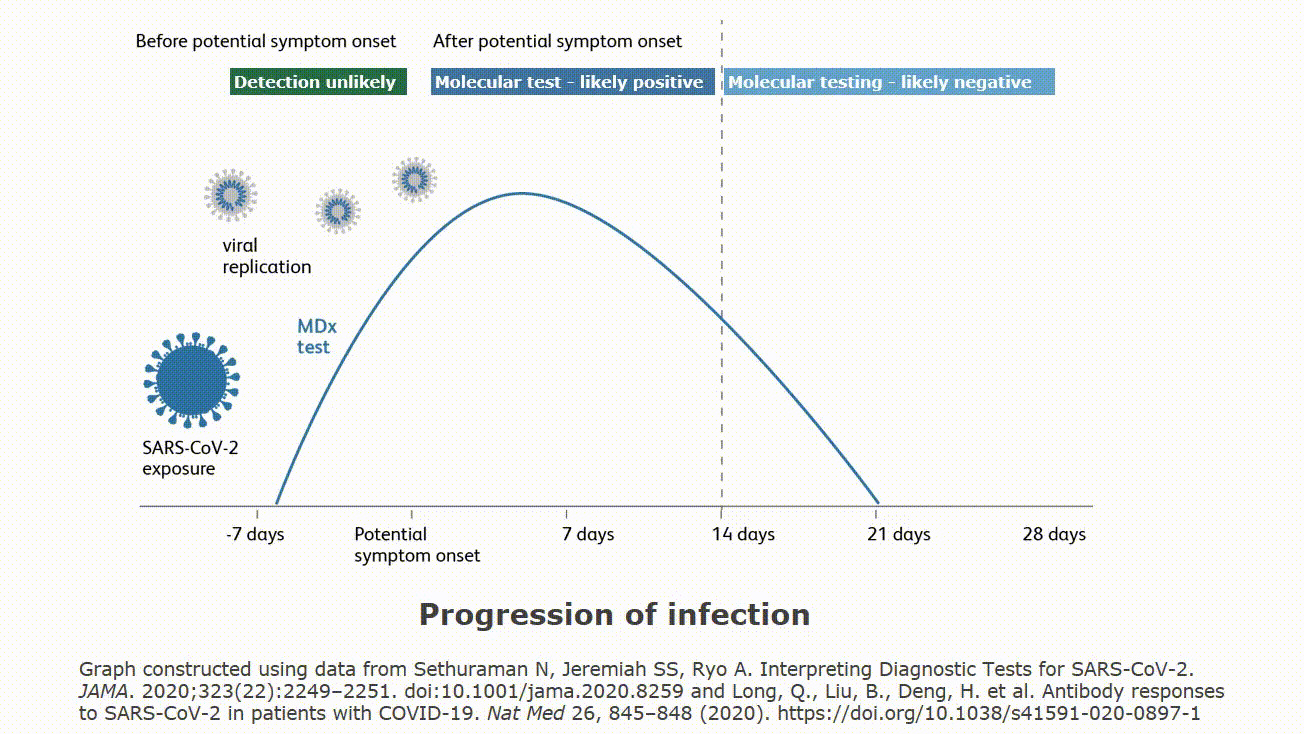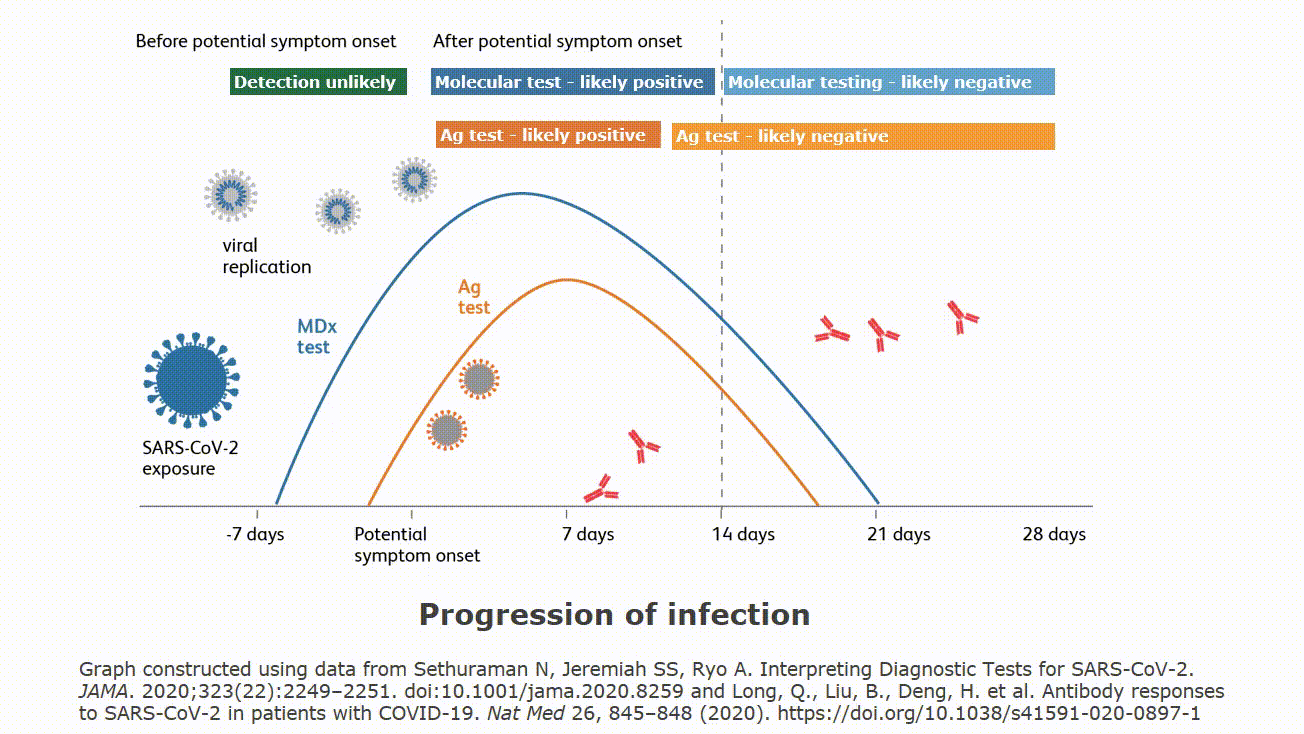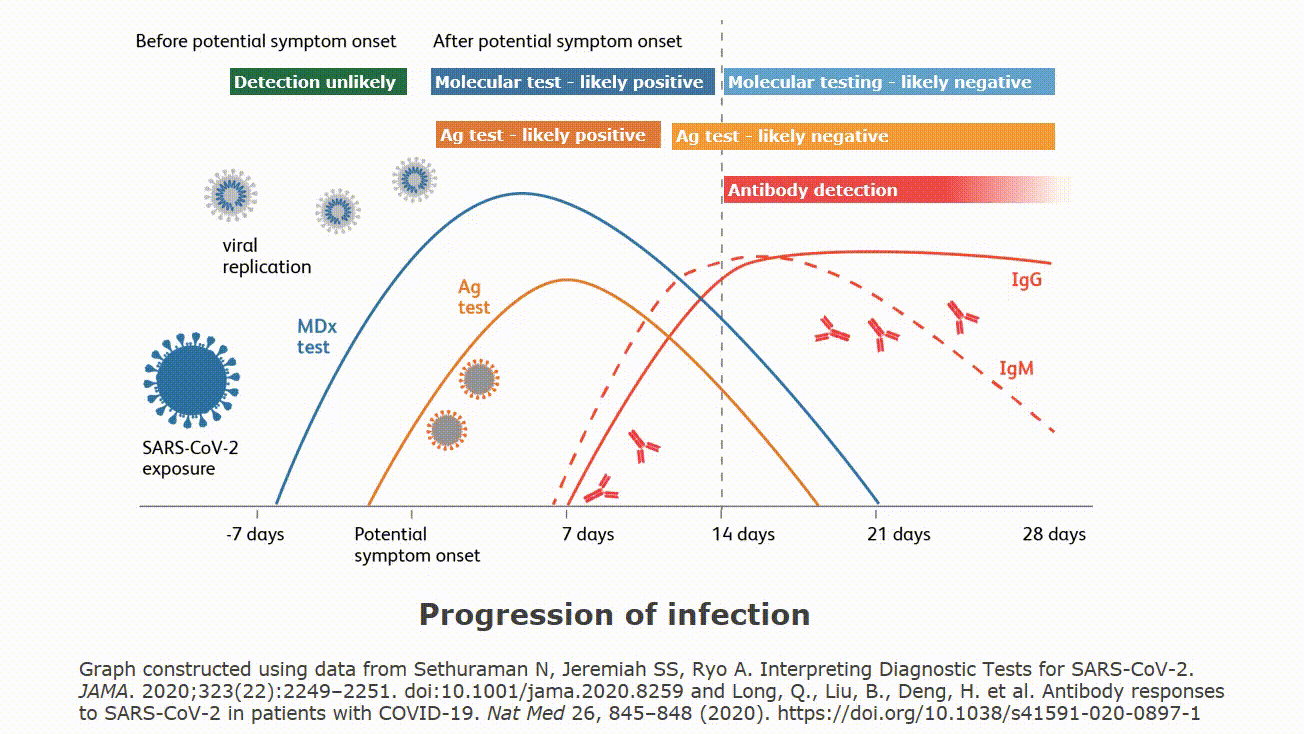
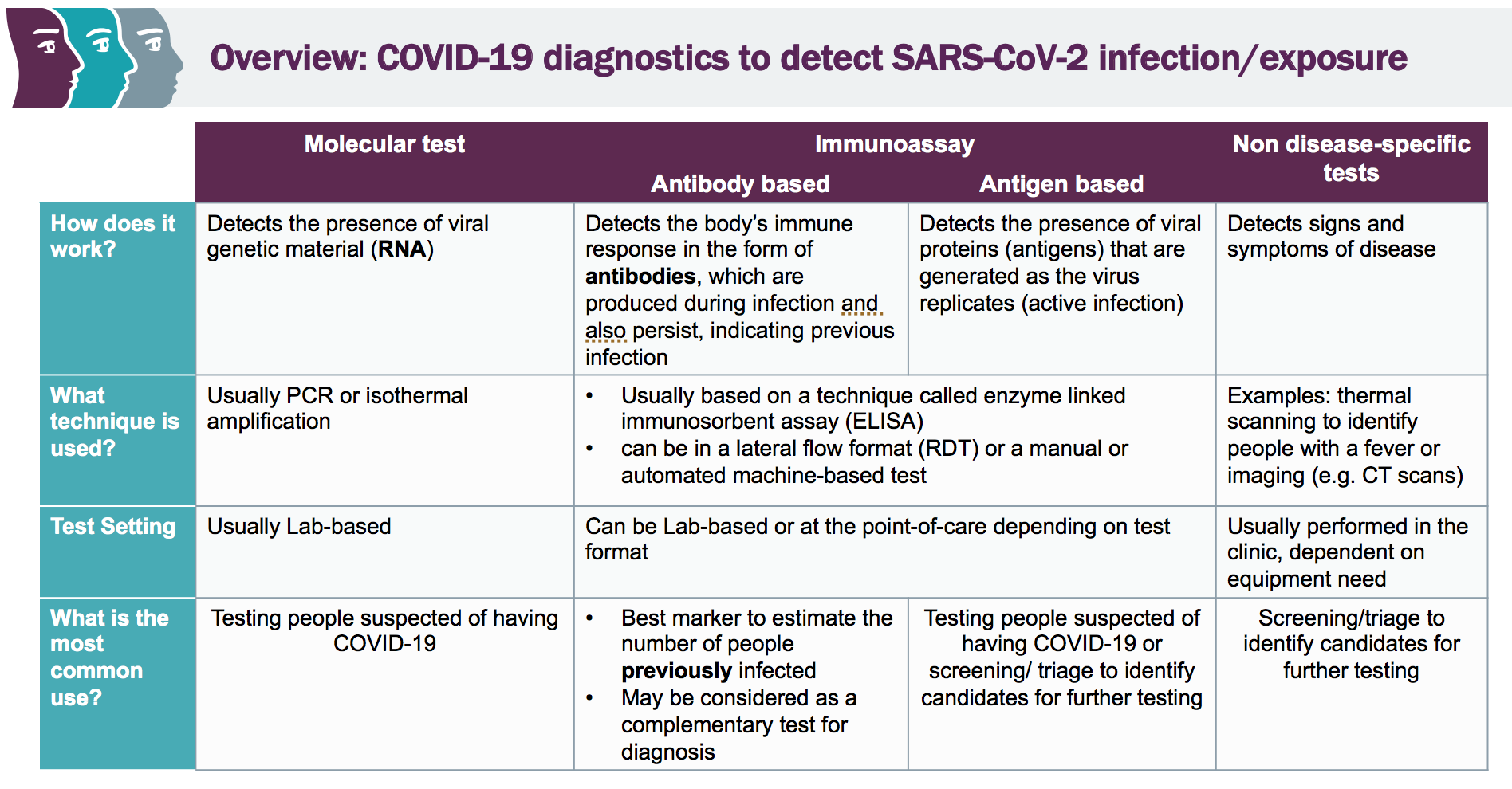
Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus genome then amplifying that portion until there s enough for detection.
Awaiting response from manufacturer.
Point of care antigen test devices on july 14 centers for medicare and medicaid cms announced an initiative to distribute rapid point of care poc antigen covid 19 testing devices to nursing homes across the country.
Molecular point of care testing for covid 19 offers healthcare workers rapid results in more settings where people show up for care.
Food and drug administration issued an emergency use authorization eua for the first serology antibody point of care poc test for covid 19.
The fda issued the first emergency use authorization for a point of care covid 19 diagnostic for the cepheid xpert xpress sars cov 2 test.
Point of care test.